-
Latest
 Feb 03
Feb 03
Cryopreservation Innovation in Biobanking
In biobanking, cryopreservation is a technique used to keep biological material at very low temperatures, usually -150 degrees Celsius (-238 degrees Fahrenheit). For cells, tissues, and other biological components to remain viable and effective throughout time, this process is necessary. Technological advancements in cryopreservation have proven essential to increasing the efficacy and efficiency of biobanking
-
Latest
 Jan 05
Jan 05
Blockchain Technology in Biobanking
Biobanking, a critical component of modern healthcare and research, involves the collection, storage, and management of biological specimens and associated data. Ensuring the integrity, security, and transparent flow of information within the biobanking ecosystem is paramount for maintaining the credibility and reliability of scientific research and medical advancements. In this context, blockchain technology emerges as a disruptive force, offering a decentralized, secure, and transparent framework that has the potential to redefine the way biobanks handle data and samples.
-
Latest
 Dec 25
Dec 25
Exploring Innovative Research Biobanks for Rare Diseases
Biobanking plays a pivotal role in advancing rare disease research by serving as a repository for valuable biological samples. These repositories, known as biobanks, store diverse specimens such as tissues, blood, and genetic materials from individuals affected by rare diseases. The collection and preservation of these samples contribute to a comprehensive understanding of rare diseases, facilitating breakthroughs in diagnostics, treatment development, and personalized medicine. In the context of rare diseases, where patient populations are often small and geographically dispersed, biobanks play a crucial role in aggregating valuable data and fostering collaborative research efforts. By centralizing these biological resources, biobanks enable researchers to access a larger pool of samples, accelerating the pace of scientific discovery and potentially uncovering insights into the underlying mechanisms of rare diseases. As a result, biobanking emerges as a cornerstone in the pursuit of effective solutions for the diagnosis and treatment of rare diseases.
-
Latest
 Nov 28
Nov 28
Ethical and Regulatory Considerations in Biobanking
Biobanking, the systematic gathering and preservation of biological specimens, is pivotal for advancing biomedical research, personalized medicine, and drug development. However, this practice brings forth significant ethical and regulatory quandaries that demand careful consideration. Balancing the imperative to advance scientific knowledge with the protection of donor rights and interests is paramount. Ethical concerns include informed consent, privacy, and the potential for stigmatization. Robust regulatory frameworks must govern sample acquisition, storage, and usage to ensure transparency, security, and adherence to ethical standards. Striking this balance not only upholds the integrity of biobanking but also sustains public confidence in the research enterprise. Upholding principles of autonomy, beneficence, and justice is essential to navigating the ethical terrain of biobanking responsibly.
-
 Oct 26
Oct 26
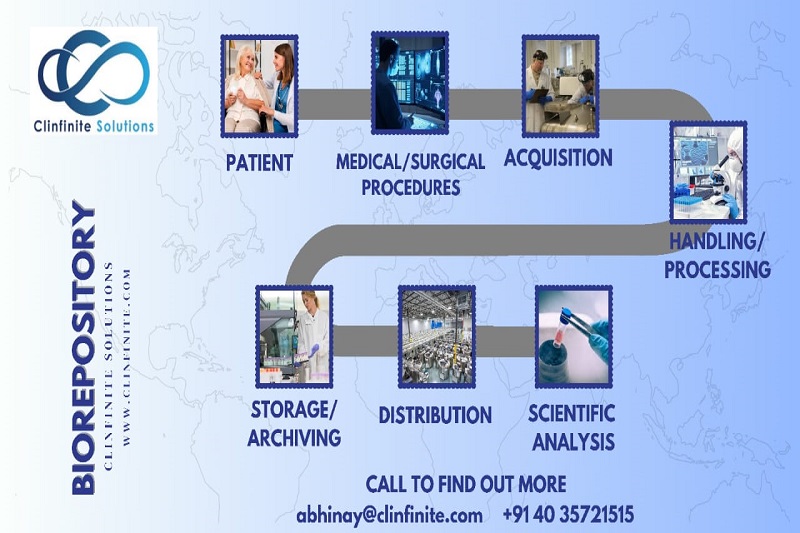
Bio-Specimen Sample Collection and Processing: A Comprehensive Guide
Bio-specimen samples are biological materials, such as blood, urine, saliva, tissue, and cells that are collected from humans or animals for research or diagnostic purposes. The accurate collection and processing of bio-specimen samples is essential to ensure the quality and reliability of the results obtained from subsequent analyses.
-
 Sep 22
Sep 22
Explain the meaning of biobanking and its importance
Biobanking, a word gaining popularity in scientific fields, is at the forefront of revolutionary research and medicinal advancements. This article looks into the complexities of biobanking, its historical evolution, the various types of biobanks, the essential processes involved, and its vital role in defining the future of medical research.
The collecting, storage, and processing of biological samples for research purposes is known as biobanking. Blood, tissue, DNA, RNA, and even living cells can be used as samples. Biobanks are essentially archives for these precious resources, which are made available to scientists researching a wide range of diseases and health situations.
-
 Sep 14
Sep 14
FFPE and Frozen Biospecimen Samples
FFPE (Formalin-Fixed Paraffin-Embedded) and frozen biospecimen samples are vital resources in medical research. FFPE samples involve preserving tissues in formaldehyde, followed by embedding in paraffin wax, making them suitable for long-term storage. These samples are advantageous for retrospective studies, as they retain structural integrity.
On the other hand, frozen biospecimen samples are snap-frozen and maintained at ultra-low temperatures. This method preserves biomolecules like DNA, RNA, and proteins, making them ideal for molecular analysis. However, frozen samples may degrade over time, requiring stringent storage conditions.
Researchers choose between FFPE and frozen samples based on their specific research goals. FFPE offers histological insights, while frozen samples are preferred for molecular investigations. Both sample types are invaluable in advancing our understanding of diseases and developing diagnostic and therapeutic strategies. -
 Sep 13
Sep 13
How Clinfinite Solutions Offers Innovative and Reliable Bio-Specimen and Bio-Repository Solutions for Clinical Research
Clinfinite Solutions is at the forefront of revolutionizing clinical research through its innovative and reliable bio-specimen and bio-repository solutions. By combining cutting-edge technology with a deep understanding of the clinical research landscape, Clinfinite Solutions ensures that researchers have access to high-quality, well-preserved bio-specimens for their studies. Through advanced storage and tracking systems, they safeguard the integrity of valuable biological samples, offering researchers peace of mind. Their commitment to compliance and quality control guarantees the reliability of data generated from these specimens, ultimately enhancing the credibility of clinical research outcomes. Clinfinite Solutions' dedication to innovation and reliability is paving the way for groundbreaking discoveries in the field of healthcare, making it an indispensable partner for researchers worldwide.
-
 Aug 28
Aug 28
Exploring the world of Biobanking: Unlocking Secrets of Human Health
In the realm of medical research, the practice of biobanking stands as a beacon of hope, holding the promise of unravelling the mysteries of human health and disease. Biobanking, the meticulous collection, storage, and management of biological samples and associated data, has revolutionized the way researchers investigate the intricate mechanisms underlying various medical conditions. Let's delve into this captivating world, where tiny samples can hold immense potential. At its core, biobanking involves the careful preservation of a diverse array of biological materials, including tissues, blood, DNA, and bodily fluids. These samples, along with detailed information about donors' medical histories and genetic profiles, create a treasure trove of resources for researchers to tap into. This reservoir of data fuels studies ranging from cancer genetics to neurological disorders, ultimately paving the way for groundbreaking medical breakthroughs. Ethics and legality are fundamental cornerstones of biobanking. Informed consent from donors is paramount, ensuring that their contributions are made willingly and responsibly. Safeguarding privacy and adhering to stringent regulations ensure that this invaluable data is handled ethically and transparently. One of the most significant impacts of biobanking lies in its potential to usher in an era of personalized medicine. By analysing genetic markers and understanding individual variations, scientists can tailor treatments to suit a patient's unique biological makeup. This revolutionary approach holds the promise of more effective treatments with fewer side effects, heralding a new dawn in healthcare. Moreover, biobanks foster collaboration among researchers and institutions across the globe. This collaborative spirit enables scientists to access a wealth of samples and data, transcending borders to drive discoveries and advancements that benefit humanity at large. In a world where diseases continue to challenge medical professionals, biobanking shines as a beacon of hope. Its ability to provide researchers with essential resources for understanding disease mechanisms, discovering biomarkers, and developing targeted therapies is a testament to its transformative potential. As biobanks continue to grow, supported by ethical considerations and rigorous quality control measures, their impact on medical science will undoubtedly be felt for generations to come. In conclusion, biobanking stands as a bridge between the mysteries of human biology and the potential for medical breakthroughs. Through ethical practices, global collaboration, and meticulous sample preservation, biobanks pave the way for a healthier future. As we unlock the secrets of human health, we stand on the brink of a new era in medicine—one where precision and personalized care are not just aspirations, but tangible realities.
-
 Feb 03
Feb 03
1 Complete General Explanation of the Process to Approve Drugs
The approval process of the drug is important because once such drugs are launched in markets, they reach consumers as safe and effective when launched in markets because the process is stringently tested and analyzed by regulatory bodies. This stage can also be even more focused on public health protection. While it took over many years of life to research and develop, it would only take one approval of a drug to be of the utmost importance in keeping the patient away from danger and giving them treatments that could perhaps save their lives. This paper will serve in describing various steps concerning how drug approval occurs. All of this, however may be true since the whole process that goes from preclinical testing up to post-market surveillance is crucial.
-
 Feb 03
Feb 03
Biostatistics: A Critical Discipline in Medical Research and Public Health
Biostatistics is the application of statistical principles to the interpretation of bios, clinical, and public health data. It is one important tool in the planning, processing, and interpretation of data in medical research. Included here are no doubt epidemiological and clinical trials. Biostatistical analysis will assist researchers in receiving valid results from complicated information, which will allow them to perceive patterns of illness, discover new medical therapies, and make available a better result in terms of public health. This article gives an indepth introduction into biostatistics, with emphasis on the fundamental concepts of the discipline, main techniques, widespread applications, and ever greater relevance in today's data-intensive science.
-
 Feb 03
Feb 03
Understanding the Blood Collection Process
Blood collection could be described as an integral component of modern-day diagnostic procedures and treatments. Whether it occurs at scheduled times-for example, regular tests, transfusions, or investigations-proper blood collection will always ensure the right result for the patient and health personnel. That is why this paper walks through step-by-step details of collecting blood, its steps, and methods together with what is crucially important-its best practices concerning effectiveness and reliability in the sphere of health.
-
 Feb 03
Feb 03
Clinical Development Solutions:Everything you need to know about Clinical Development Solutions
One of the very last stages, in a sense, before new medicines and treatments are launched on the market is clinical development. In highly controlled clinical trials, drugs, biologics, or even medical devices- step by step-are tested in detail. Such studies are conducted with the aim of ensuring maximum benefit and lowest possible risk of the product and to prove its safety and efficacy in use. Clinical development solutions for planning, conducting and finalizing clinical trials in all-rounded ways in all overlapping manners are supportive. It cuts straight to core clinical development and gives a good number of strategies and concepts for improvement without compromising compliance with regulations.
-
 Feb 03
Feb 03
Understanding the Clinical trials Process
Clinical trials are the lifeblood of medical research, for it is the only way to determine whether a new drug, therapy or device works and is safe. The useful information generated by scientists, physicians, regulators must wait until orders of magnitude later to be used in saving lives. Without clinical trials, advances in medicine would grind to a halt and unproven-and therefore potentially hazardous-practices would continue to delay evidence-based healthcare.This post will talk the Clinical Trials Phases, Components of it, Involvement of a participant, The New Additions in this which are recent innovation & Safety Intervention that a patient should know in current Trial Studies.
-
 Feb 03
Feb 03
Contract Research Organizations (CROs): Embracing the Future of Clinical Research
Contract research organizations, or CROs, will be an integral part of the future for the biopharmaceutical, biotechnology, and medical device industries. They are a partner that should be a perfect complement to clinical research, giving companies the ability to develop new treatments much faster and more cheaply than has been thought possible before. Clinical research organizations provide knowledge, tools, and operational capabilities that are required in handling this increasingly demanding regulatory environment and strong global competition. This paper attempts to provide an all-encompassing analysis of the operations, benefits, services, challenges, and future prospects by sector in CROs.
-
 Feb 03
Feb 03
General CRO Services Definition: Chemical Catalysts of New-age Clinical Trials
Contract research organisations (CROs) are, in themselves, of immense importance and have a big impact on the field of medicine research and clinical trials. CROs are supporting the pharmaceutical companies, biotechnology enterprises, and medical device manufacturers to fully comply with the intricate regulations. In this, it brings experience to carry out various stages of clinical studies so that it carries it out according to global standards, and enables the sponsors to receive revolutionary treatments to market in an accelerated manner and with efficiency. Below is an outline of all the main CRO services, advantages that they bring to sponsors, their role in the drug development process, and how they help in innovations in clinical research.
-
 Feb 03
Feb 03
Depth Explanation on Data Management Services
The world today calls for the inflow of enormous amounts of information into the enterprise from different sources. Data management is the backbone derived for operational effectiveness, compliance, and informed decisions. Multiple data management processes, tools, and techniques ensure levage of data to its maximal extent within an organization through various means. This article reflects some of the major elements of the importance of data management services and its best practices in the area of implementation.
-
 Feb 03
Feb 03
Comprehensive Development Plan for Global Solutions for Development
Development solutions would focus on the economic, social, and environmental problems that the countries of the world face. In a time when populations grow and resources become scarcer, it is now more important than ever to produce sustainable, inclusive, and effective methods. From educational reforms to infrastructure developments, they can be vast with different reasons as to what specific issue the region may call for in terms of its particular needs. The article aims at giving a view on some of the most important sectors—in terms of infrastructure, technology, education, and environmental sustainability—and how development solutions would likely determine a huge influence in them.
-
 Feb 03
Feb 03
Innovative and Healthcare Solutions World
The most important work of those companies which design and develop and manufacture a number of medical devices useful in diagnosis, treatment, and prevention of a wide variety of diseases. It is absolutely very important to know and identify the best medical device company in your neighborhood as a researcher or as an expert practitioner or as a patient to avail of the latest technology and for better patient care. These companies manufacture a number of products that include basically wearable health monitors, prosthetic products, surgical instruments, and diagnostic equipment among many others. Some of the types of medical device companies what they do, and how to find the best near you
-
 Feb 03
Feb 03
Medical Writing: An Important Role in Medical Documentation
The medical writers specialize in writing clear, concise, and accurate documentation for use in a wide range of healthcare, clinical research, drug, and regulatory contexts. Qualified medical writers have been in huge demand as the regulatory environment is becoming ever more stringent and the health care landscape increasingly complex. Medical writing connects the scientific community with healthcare providers and regulatory organizations in the sense that complicated material is communicated to a variety of audiences. The medical writer's role is far from simple documentation. This professional needs to be highly knowledgeable about clinical procedures, research methods, legal requirements, and, of course, medical terms. They can create a range of writings. These can include the clinical research reports, writing for patient education, journal articles, or regulatory documentation, such as clinical trial protocols and regulatory submission documents, amongst others. The paper discusses several aspects of medical writing and its value to clinical research and treatment.
-
 Feb 03
Feb 03
LabCorp's commitment to the development of clinical trials: a tower of precision and innovation in global health
LabCorp is one of the leading companies in the world for life sciences. It plays a very crucial role in the forward movement of clinical trials. The company capabilities range from designing a trial to recruiting a patient, performing clinical laboratory services, and data management support in order to comply with the regulatory requirement. An integrated approach within the trails in LabCorp ensures there's always a benefit that will be effective to be produced. This, in turn, produces drugs much quicker and leads to better patient results.
-
 Feb 03
Feb 03
Regulatory Submissions: A Step-by-Step Guide
All pharmaceutical companies that intend to introduce new drugs or drugs in a country have to seek regulatory approval from the appropriate agencies before allowing their entry into the public marketplace. These agencies use such applications to check whether the product passed such standards of safety, efficacy, and quality before it gets the nod to enter the public marketplace. In as much as this process is useful for public protection, it helps in an attempt to cut undesirable diffusion to public of valueless products. This paper details the process of submission, relevance, forms of submissions, significant procedures, common problems, and best practices upon making the submission go well.
-
 Feb 03
Feb 03
Research Companies in My Area: Finding the Best Partner for Innovation
Research firms help innovations and developments in all industries by providing services that are required to assess innovative ideas, design next-generation technology, and solve complex problems, whether it is an academician, a startup owner or a business leader. As research companies have specialized in the most niche areas-from market surveys and technological development to medical research and environmental science-research services demand the most in-demand product over the last few years. This article talks about the various types of research firms that are there, their functionalities, and how to find the best local partner.
-
 Feb 03
Feb 03
Understanding the Blood Collection Process
Blood collection could be described as an integral component of modern-day diagnostic procedures and treatments. Whether it occurs at scheduled times-for example, regular tests, transfusions, or investigations-proper blood collection will always ensure the right result for the patient and health personnel. That is why this paper walks through step-by-step details of collecting blood, its steps, and methods together with what is crucially important-its best practices concerning effectiveness and reliability in the sphere of health.
-
 Feb 03
Feb 03
Serum Sample Collection: A Full Review
Serum sample collection is relatively simple in most of the medical research as well as other operations performed in the laboratory. The outcome of the test would heavily rely on the quality of the serum sample. The manner of sampling and handling might expose the possibility of altering the trend of diagnosis and most likely treatment of the patient. In this paper, we will clearly indicate procedures followed, preparation requirements, and proper procedures for serum sampling collection. We will discuss general problems which often come with collection and how to address them to ensure output from your facility is the best.
-
 Feb 03
Feb 03
Guide and Everything You Know You Need to Know About Urine Samples Collection
The process of taking urine samples is one procedure that primarily applies in monitoring and diagnosis in hospitals. The test is performed from the patient's sample, from which one can run several medical conditions. Getting proper results entails determining whether proper technique is being used. This paper will describe step-by-step how a sample of urine is taken. There are several techniques that apply when taking urine samples; first, among the greatest safety measures to be observed, and then why or what could affect the results.
-
 Feb 03
Feb 03
Understanding Urine Specimen Collection Procedure
Urine specimens collection is a basic medical testing procedure. Many systemic and localized diseases, including kidney disease, urinary tract infections, and others, can be diagnosed through urine testing. Proper urine specimen collection alone can guarantee reliable test results that are more important for diagnostic and therapeutic purposes. Various methods of urine collection are available depending on the type of test to be carried out and the condition of the patient. The knowledge of what protocols should be applied on the collection of urine samples will ensure optimal performance in patients and healthcare providers in test procedures.
-
 Feb 03
Feb 03
Clinical Development Services: Where Innovation Meets Patient Care
The health care sector's clinical development services provide the framework essential for the development of a new medical therapy from the laboratory to clinical application. Such services range from data management, regulations on the use of patients in clinical trials and more. The critical and challenging aspects relating to clinical development services will form the crux of this essay to consider the various perspectives that drive home the critical aspects and challenges of this dynamic 21st century health care context.
-
 Feb 03
Feb 03
Understanding Biospecimen: Importance, Types, and Ethical Considerations
Biospecimens are biological materials for example, blood, tissue, or DNA, which are collected from living things, mainly humans, for the purpose of scientific study. These resources are of humongous dimensions in the pursuit of our bodies of medicine, design of novel therapeutic interventions, and provision of individualized health care. When the biospecimens are examined, the researchers will get information about different kinds of diseases, hered- itary traits, and drug reactions, and they can develop ways of diagnostic and treatment results that are more effective.
-
 Feb 03
Feb 03
2 Unlocks Future with Biometric Solutions
The security, authentication and identification technologies of this present world is reliant on the biometric technologies. This technology has exploited particular characteristics that can be found in biological materials thereby providing incomparable accuracy and ease in a high number of sectors. Applications for these technologies-the list includes facial recognition, fingerprint recognition, and all points in between-between industries range from government, finance, and health to name a few. This essay is deep into the many parts of biometric solutions to explore their relevance, uses, and prospects.
-
 Feb 03
Feb 03
Regulatory Medical Writing: An Essential Component of Pharmaceutical and Clinical Research
Regulatory medical writing forms a platform of biotechnology and pharmaceutical industry. It says structuring valid documents, being all comformant, well prepared at all moments to submit to regulatory authorities and the entire clinical data, for which, the findings of trials studies, safety profile of drugs are displayed lucidly and time-effectively. Regulatory writers help the pharma company to get a nod for new medicines, treatment, and medical devices in the form of proper assimilation of scientific and medical data with well-balanced reports. This paper discusses application and importance and best practices of regulatory medical writing in the clinical research activity.
-
 Feb 03
Feb 03
Blood Collection: Introduction and Best Practices
Blood collection is the lead process in medical diagnosis and treatment and forms the basis for most tests and assessments. Whether routine check-up or more specialized diagnostic procedure, how accurately blood would be obtained would mean valid results. And thus, there must be several steps in the process from the preparation of patients to specific techniques directed towards minimum contamination and therefore minimum risk both for the patients themselves and for those carrying out treatment.
-
 Feb 03
Feb 03
The Role of Clinical Research Organizations (CRO) in Clinical Trials: A Comprehensive Overview
Clinical Research Organizations, or CROs, play a very fundamental role in the dynamic evolution of the clinical trial environment. CROs are at the foundation of clinical research, as they provide pharmaceutical, biotechnology, and medical device companies with specialized knowledge about operating trials, regulatory compliance, and accelerating drug development procedures. This paper discusses the role of CROs in clinical trials, benefits, difficulties, and ways they favor the completion of clinical studies.
-
 Feb 03
Feb 03
Exploring the Landscape of eClinical Solutions
The clinical trial industry is fast being altered by the need for more effective and data-driven methods. That's because change in clinical trial management, in contract research organizations, and pharmaceutical corporations results from integrating technology into the trail process through eClinical solutions. Modern drug development requires eClinical technologies increasingly because they allow improvement in productivity, cutting expenses, and ensuring better regulatory compliance. This paper discusses the field of eClinical, its elementary components, advantages and difficulties, and possible directions.
-
 Feb 03
Feb 03
Exploring the Role and Impact of Contract Research Organizations (CROs) in the Pharmaceutical and Biotech Industries
Biotechnology and pharma, today, above all else, cannot be done without Contract Research Organizations or CROs. CROs offer services that include post-market surveillance and clinical trials, preclinical testing, and drug development that are basically helpful for conducting successful and efficient R&D procedures. Stricter regulations and a greater need to bring new treatments into the market quickly also speak of the importance of having CROs onboard. This article will therefore present about definition, types, role of CROs in drug development, the advantages and disadvantages as well as its future development in the industry.
-
 Feb 03
Feb 03
Comprehensive Guide to Specimen Collection
Specimen collection is one of the basic procedures used in a clinical and laboratory setting. It acts as the basis on which proper diagnoses are acquired and patients are treated with maximum efficiency. The knowledge of many techniques and guidelines associated with specimen procurement may significantly enhance the quality of test results. This paper discusses the importance of proper specimen collection, techniques used, problems encountered, and practice recommendations for clinicians.
-
 Feb 03
Feb 03
Understanding the Fundamentals of Clinical Research
Clinical research is a subgroup of medical science which is involved in several activities, treatments, and diagnostic techniques in an organized manner for the betterment of the healthcare outcome. It directly affects patient care, treatment regimens, and health policy publicly and is important to ensure safety and effective translations of medical inventions. In this paper, we will discuss the fundamental components, phases, and implications of clinical study, which are enlightening to the nature of how the scientific input may drive medicine.
-
 Feb 03
Feb 03
Clinical Research Organizations: An In-Depth Exploration
CROs form very central organizations in the pharmaceutical, biotechnology, and medical device industries. Clinical trials are a primary feature of new treatments, drugs, and medical device development. Thus, essentially, the role of a CRO derives from ensuring clinical trials are conducted by regulatory requirements, that are scientifically valid, and completed within time. They offer expertise and resources that would be required to help the sponsors-often pharmaceutical or biotech companies provide their services in the efficient conduct of clinical trials. The more complicated medical research is the greater the need for the kind of services a CRO offers. This article attempts to explore the world of CROs, focusing on their roles and types of services offered, and the challenges that arise from them. We also tell their significance in the healthcare industry, the benefit of creating an organizational structure for their advantage, and the future of such vital organizations.
-
 Feb 03
Feb 03
Medical Devices Industry: A Comprehensive Overview
The modern medical devices industry assumes the critical role of providing healthcare systems with much-needed diagnoses, monitoring, and treatment of a wide range of health conditions. Increasing levels of technological advancement spur growing needs for new and more efficient medical devices, and this undoubtedly changes the mode of health delivery. Thus, this article seeks to cover a comprehensive review of the medical devices industry, discussing its market landscape, key technologies, regulatory environment, and future trends
-
 Feb 03
Feb 03
Navigating Cancer Clinical Trials: A Comprehensive Guide
There is no other way to improve cancer treatment and understand the disease dynamics except through clinical trials. They progress scientific knowledge, but simultaneously give patients access to advanced therapies. It is regarding the phases, kinds, and importance of clinical trials on cancer that this paper explores their complicated nature.
-
 Feb 03
Feb 03
Understanding Covance Clinical Trials: A Gateway to Innovation in Healthcare
Clinical trials resulted in one of the first movers in the drug development services, Covance. It makes new treatments, which could prove capable of altering the very face of patient care, feasible by closing science research gaps as well as its actual application. On this page, the complexities of clinical trials with Covance are evaluated, from the types to the importance and contributions towards the improvement of healthcare.
-
 Feb 03
Feb 03
The Complex Journey of Drug Development: From Concept to Market
The whole process of drug development is very complex and takes much time, bringing scientific discovery into medicine safety and efficacy. Such a process will involve rigorous testing, regulatory oversight, and a lot of financial cost. Knowing the steps in drug development allows us to understand the innovations and challenges that have led to the current medicine.
-
 Feb 03
Feb 03
CRO Pharma: A Comprehensive Overview
The development stages, current state, and prospects of CRO Pharma give the background information of this information. These organizations, especially Contract Research Organizations (CROs), ease and greatly facilitate the quest for drugs in this challenging time when the medical device sector is changing fast. CRO Pharma stands out from the rest of these organizations and is one of the leading companies in the sector.
-
 Feb 03
Feb 03
Understanding ECOA Clinical Trials: Enhancing Patient-Centered Research
Patient perceptions and technological advancements are being increasingly integral components in the dynamic medical science field. Among these, Electronic Clinical Outcome Assessments (ECOA) feature importantly in this transformation: ECOA offers the tools that support allowing patients to be a part of the clinical trials and to collect data. This article discusses how clinical trials related to ECOA give rise to more efficient and patient-centered solutions in healthcare.
-
 Feb 03
Feb 03
The Power of CRO Research: Strategies for Success
Optimization of conversion rates (CRO) is undoubtedly among the most important tactics that can support the improvement of marketing efforts effectiveness in this digitalized e-commerce world. Research forms the base for a perfect CRO. This article will highlight the importance of CRO research and the methods used to implement it along with some best practices to optimize a conversion rate.
-
 Feb 03
Feb 03
Understanding the Different Types of Drugs: A Comprehensive Guide
Drugs have highly impacted society and culture in nearly every way, affecting human health to a good extent. While some drugs are prescribed for the treatment of specific diseases, the others come under abuse or recreation, which leads to addiction and other effects. The substance use is complex, and a sense of how the substances are classified based on their effects, legality, origin, and uses is important to awareness about substance use. This page gives the reader an all-around comprehensive review of the many drug kinds, their classifications, and the effects of drug usage, hence giving the student a proper perspective on this major topic.
-
 Feb 03
Feb 03
Understanding Preclinical CROs: A Comprehensive Guide to Contract Research Organizations
The preclinical CROs play a very crucial role in the pharmaceutical and biotechnology development sectors. They offer third-party outsourced services to companies on issues related to the development or discovery of drugs, especially before the initiation of clinical trials. Preclinical CROs offer experience, state-of-the-art technology, and cost-effective procedures that reduce time and cost involved in the development of new drugs. This page will help readers to gain more knowledge of preclinical CROs, and also be aware of the role played by them, importance, and different stages of preclinical research.
-
 Feb 03
Feb 03
How Healthcare Technology Companies Are Transforming the Future of Medicine
They're using latest health technologies to aid patient results, hasten the pace of treatment, save costs, and lead in transforming the health industry. From making wearable technology for continuous health monitoring to building electronic health record systems, these businesses have become inevitable in providing modern healthcare. They design innovation in data analytics, telemedicine, artificial intelligence, and many other aspects, which make health care possible in accessible and personal ways. This paper explores the emerging phenomenon of health care technology companies, their core focus areas, challenges they face, and possible implications for healthcare in the future.
-
 Feb 03
Feb 03
Understanding Biological Samples: A Comprehensive Guide
Majorly, biological samples are crucial in biomedical research and diagnostics while also in the development of new treatments. Through such samples, one will be able to access the cellular and molecular functions taking place in a body. These samples may, therefore, include blood, tissues, saliva, among others as well as other biological components. Biological samples have actually formed a basis for most researchers to investigate diseases, find biomarkers and consequently develop personalized therapy strategies. However, some samples of this particular type pose certain specific challenges in terms of management and maintenance of such samples, including data integrity, preservation techniques, and ethical issues. The report therefore provides a comprehensive review of biological samples that involve types, applications, and difficulties in gathering, storing, and analyzing them.
-
 Feb 03
Feb 03
3 Regulatory Writing: A Critical Component of Drug Development and Approval Processes
In the biotechnology and pharmaceutical industries, regulatory writing is the name of the game. It is the art of creating all the documentation required to obtain approval for pharmaceutical, biologic, and medical device development and marketing. The European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), and other global health authorities regulate most of the requisites that these documents should be in and follow. The writing of regulations is very specialized, requiring that the expert have a deep understanding not only of regulatory demands but also of scientific ideas. This article reviews the importance of regulatory writing, its key elements, and its role in the launch of safe and efficient products in the market.
-
 Feb 03
Feb 03
The Role of Biometric Services in Enhancing Security
With biometric services, identification, security, and data protection have all been dramatically changed. Biometric technologies as compared to the more conventional methods of password or ID cards relies on unique biological features that include voiceprints, iris patterns, fingerprints, and facial recognition. This system, therefore, gives better security with more accuracy in procedures for access and identification that are difficult to hack. In this article, several kinds of biometric services have been placed together with their uses, difficulties, and prospects for biometric technology.
-
 Feb 03
Feb 03
The Role of Biometrics in Contract Research Organizations (CROs)
Contract Research Organizations (CROs) play an important role in the fast area of clinical research since these organizations make it easy for new treatments and drugs to be found. Biometrics is a game-changer because clinical study is becoming increasingly complex and because more efficient methodologies are required. This paper pursues the significance of biometrics in CROs, its uses, benefits, challenges, and future scope for biometric technologies in clinical research.
-
 Feb 03
Feb 03
Leading Contract Research Organizations: Pioneers in Clinical Research
Contract Research Organizations, the future of this fast-moving biotechnology and pharmaceutical industry, are a must in launching new therapies onto the market. The services availed of by companies aid in the development and commercialization of pharmaceuticals, simplify the process of research and meet all legal requirements. In this paper, some of the world's best CROs are presented, describing their innovations, fields of specialization, and contributions to the clinical research sector.
-
 Feb 03
Feb 03
The Integral Role of Research in Clinical Medicine
In contemporary healthcare, research and clinical practice are two sides of the same coin. Research seeks new knowledge through inquiry and testing, while the clinical application is the application of such knowledge to care for patients. The essay delineates the relevance, interdependence, as well as emerging trends that are changing healthcare provision.
-
 Feb 03
Feb 03
Clinical Operations: The Backbone of Successful Clinical Trials
Clinical operations play a crucial role in the health industry and therefore depend on clinical trials whereby new drugs, treatments, and medical equipment are tested. These include coordinating, conducting, and managing clinical research, and they must agree with the legal directives and moral obligations. Relevance, procedures, difficulties, and possible paths of clinical operations are analyzed in-depth in this article.
-
 Feb 03
Feb 03
Innovative Clinical Solutions: Transforming Healthcare Delivery
It is very important in the era of rapid advancements in technology; outcomes for the patients, smooth operations, and total quality care are very important. It would call for growing needs for clinical solutions in many advanced clinical solutions that are changing the healthcare industry. Many of the possible uses, advantages, and future possibilities for the patients and the providers will be found in this article.
-
 Feb 03
Feb 03
Data Management: Unlocking Information's Potential
Data is the most critical asset that an organization owns in the modern world of technology. In all fields, from healthcare to banking, retail, or education, the proper management of data is now an essential necessity. Rules, processes, and technologies involved in collecting, storing, and processing data properly and securely fall under data management. With all types of data being collected by companies, the information must be valid, secure, and easily accessible. Therefore, this article reviews best practices, explores data-management concepts, and delivers a roadmap for streamlining data-handling processes within your company
-
 Feb 03
Feb 03
The Critical Role of Clinical Research Associates in Drug Development
Clinical trials have been considered as the highest critical elements in the introduction of drugs, medical equipment, and treatments in the market whose efficiency depends mainly on the efficiency of the CRA. Some of their duties include monitoring the sites for clinical trials and ensuring that there is compliance with the regulations and factors that contribute towards improving health care. It would discuss all issues concerning the CRA role, including what obligations and challenges the role entails, amongst other things necessary to succeed in this continually changing field.
-
 Feb 03
Feb 03
Understanding CROs: Contract Research Organizations in the Clinical Research Industry
Contract research organizations are as indispensable to the pharmaceutical industry as they are to the world of clinical trials. A CRO is essentially an organization that accelerates the processes of drug and treatment development through specialized services provided to biotechnology, pharmaceutical, and medical device companies. An understanding of what a CRO is, its scope of operation, and its role in the big picture of clinical research can give insight into the subtleties of today's drug development. This article, it has been able to discuss the different dimensions of CROs and the introduction of those in the market with their significance during clinical research and why they cannot be dispensed with the healthcare system at present times.
-
 Feb 03
Feb 03
Understanding The Principle of Blood Collection
Venipuncture, or the collection of blood, is one of the importance aspects of medical research, diagnosis, and treatment. Venipuncture involves the puncture of a vein, commonly in the arm, to obtain blood for diagnostic purposes and for the administration of appropriate medication. Generally, the process calls for great skill, precision, and safety precautions. Knowing the basic principles of blood collection is one of the safety precautions for the patient, along with the accuracy of test results. This text discusses that area in detail while incorporating much-needed information that includes equipment usage, preparation of patients, and techniques that build on this critical medical procedure.
-
 Feb 03
Feb 03
Comprehensive Overview of Medical Writing Services
Medical writing services have become an important source of support for the pharmaceutical and healthcare industries. Such services involve putting together science-compliant documents-clear, accurate, and produced for the purposes of disseminating research findings, clinical trial data, regulatory requirements, and other resources for medical education. This is very much needed to be used for marketing, regulatory submissions, and patient education, among other reasons. This article discusses aspects of the medical writing service, including types, procedures, and importance in the medical industry.
-
 Feb 03
Feb 03
The Role of Clinical Device Companies in Advancing Healthcare
Clinical equipment manufacturers play a crucial role in the design, production, and supply of instruments that enhance health care. These companies significantly support advancement in healthcare through supplying a large range of devices from therapeutic devices to diagnostic instruments. This paper examines the main functions of clinical equipment manufacturers, their mode of operation, and their impact on the healthcare industry. As fast as medical technology is evolving, it is important to understand how they work. This review canvasses all aspects of services delivered, challenges faced, and the potential that exists for clinical device companies into the future.
-
 Feb 03
Feb 03
Clinical Research Institutes: Pioneers of Medical Innovation
Short for clinical research institutes, CRIs are dedicated to the no man's land between laboratory-based discoveries and applications in real life. The very first act toward developing new medicines and therapies is an application in the clinic, namely a clinical trial. Contemplating the totality of clinical research will give an interesting insight into the need for clinical research for innovation in patient care as well as public health.
-
 Feb 03
Feb 03
Laboratory Sample Collection: Best Practices and Procedures
Laboratory sample acquisition is an integral part of the diagnostic process of healthcare. It plays a significant role as regards the nature of handling and transportation of samples collected; this actually affects the quality and reliability of laboratory results. The article contains the processes, importance, and challenges involved in the collection and best practices to ensure high-quality results.
-
 Feb 03
Feb 03
Comprehensive Guide to Specimen Collection Procedures
Specimen collection is an integral part of medical research and diagnosis that provides the specimens needed in the laboratory for testing. Proper procedures and procedures in specimen collection define specimen integrity, while test findings accuracy consequently affects patient care. Effective specimen collecting involves a lot of steps, factors, and best practices that are all dealt with in this article.
-
 Feb 03
Feb 03
A Comprehensive Guide to Lab Sample Collection
Specimen collection is the heart of laboratory research and diagnostic medicine. According to reports, the accuracy of a laboratory test or even its reliability in the first place depends on how samples are collected, handled, and delivered. This article gives an in-depth explanation of the process of collecting laboratory specimens, drawing down methods and best practices to ensure dependable results from quality specimens.
-
 Feb 03
Feb 03
A Comprehensive Guide to Lab Sample Collection
Specimen collection is the heart of laboratory research and diagnostic medicine. According to reports, the accuracy of a laboratory test or even its reliability in the first place depends on how samples are collected, handled, and delivered. This article gives an in-depth explanation of the process of collecting laboratory specimens, drawing down methods and best practices to ensure dependable results from quality specimens.
-
 Feb 03
Feb 03
Understanding Clinical Evaluation Reports (CER): A Comprehensive Guide for Medical Devices
Any medical device product has to go through an introduction process that includes critical clinical evaluation, and in regulatory aspects, a Clinical Evaluation Report is a document that compiles and evaluates clinical data in proving a requirement that corresponds with regulatory demands to be complied with. It describes how a high-quality CER explains importance and structures challenges associated with its compilation in the broader context of regulatory provisions for medical devices across the world.
-
 Feb 03
Feb 03
The Role of Tissue Samples in Clinical Research and Diagnostics: Key Insights
Tissue samples happen to be the most material used in medical research and diagnostics mainly because they reflect information about the composition of an organism's biota. Exploration into diseases, the learning of biological processes, and the development of remedial agents for the most part rely on tissue samples in pathology, oncology, and pharmacology. The article presents tissue samples, their varieties, and methods of collection with contributions for furtherance for new medical knowledge.
-
 Feb 03
Feb 03
Understanding The Regulatory Consulting
Regulatory consulting is the high-end provision of guidance and assistance given to organizations engaged in the very nebulous regulatory regime targeting specific industries, such as health care, pharmaceuticals, and biotechnology. The demand for regulatory consultants has been expanding due to stringent regulations continuously imposed on companies. This paper explores the importance of regulatory consulting, core elements, and future trends that define the profession.
-
 Feb 03
Feb 03
The Role of Clinical Research Organizations (CROs) in India
The Clinical Research Organizations, or CROs, play a significant role in the biotechnology and pharma sectors, especially in India. The sectors of clinical trials and research are led worldwide by CROs, enabling new drugs and therapies to be developed while ensuring that they meet marked quality and safety standards before being made available in the market. This article will critically analyze the Indian scenario on CROs with an importance of these organizations, legal environment, current issues, and emerging developments.
-
 Feb 03
Feb 03
A Comprehensive Overview of Sample Collection Methods
In many clinical and scientific domains, such as environmental science, medicine, and research, sample collecting is essential. Depending on the goals of the research, the kind of analysis needed, and the properties of the item being sampled, several kinds of samples are taken. The several kinds of sample collecting techniques are examined in this article along with their importance, uses, and best practices.
-
 Feb 03
Feb 03
Exploring the Role of CROs in Hyderabad's Biopharma Ecosystem
Hyderabad is now emerging to be known as Genome Valley and has become the hub for Clinical Research Organizations in India. Today, more pharmaceutical and biotechnology domestic and foreign businesses are being attracted to the city because of its infrastructure, competent labor, and friendly regulatory framework. The article tries to analyze the CRO scenario in Hyderabad, the role of CROs in business, the difficulties they face, and the future potential for clinical research in the region.
-
 Feb 03
Feb 03
Understanding Regulatory Compliance: A Crucial Element for Businesses
Organizations that comply with the laws, rules, standards, and specifications relevant to their business processes are considered to be in regulatory compliance. Be it any of the sectors manufacturing, finance, health care, or any other, for that matter, regulatory compliance certainly is required to avoid fines and ensure smooth operations. As the sectors become international and complicated, the regulatory compliance landscape is changing, and businesses need to adjust in accordance to come up with a stronger compliance system. To reach this conclusion, this article will explore several facets of regulatory compliance, including its importance, the role of compliance frameworks, specific industry-specific challenges, and compliance opportunities in the digital age.
-
 Feb 03
Feb 03
Exploring the Phases of Clinical Trials: A Comprehensive Guide
These are carried out in phases or stages and prove vital to advancing medical research into such new medicines and therapies to finally bring them to the market. In order that a treatment is safe, effective, and of good care to the patient, they have to be conducted in a series of phases or stages. Each step of a clinical trial serves a very specialized purpose, and it builds on the findings of the previous step, while contributing information on how the drug is actually interacting with the patient's body. Therefore, this post aims to take a closer look at numerous stages of clinical trials, starting from pre-marketing testing up to post-marketing surveillance, clarifying the goals and procedures of each stage.
-
 Feb 03
Feb 03
Biotechnology Companies: Pioneers of Modern Science and Innovation
Companies in biotechnology are the pioneers of scientific innovation. They collaborate with a variety of industries to produce goods and innovations for the better living of humans, to meet medical requirements, and to sustain life through ecological practices. In this regard, the firms manufacture drugs, agricultural products, and industrial products by utilizing biological systems, cells, and living beings. As biotechnology deals with different industries such as environmental research, healthcare, and agriculture, these businesses are necessary for the formation of modern technology. This essay is going to explore the current state of biotechnology businesses, their contribution, the difficulties that they face, and their prospects in a digital world.
-
 Feb 03
Feb 03
Understanding Clinical Trial Management Systems: A Comprehensive Guide
Clinically important trials are performed with the main objective of expanding medical knowledge and avoiding harming patients. Advanced management solutions for a trial have to be found because of the complexity of the study and legal constraints. Studies planning, monitoring, and reporting can be made more efficient by a Clinical Trial Management System (CTMS). This paper discusses characteristics, advantages, and considerations when a CTMS is implemented for assisting companies undertaking clinical research.
-
 Feb 03
Feb 03
The Role of CRO Companies in Modern Drug Development
Contract Research Organizations (CROs) are becoming one of the major players in the pharmaceutical and biotechnology industries. CROs help with very important services that add efficiency to research and accelerate clinical trials because drug development becomes more complicated and complex day by day. In this paper, I discuss the different features of CRO businesses, what role they play in drug development, and how they shape medical research in the future.
-
 Feb 03
Feb 03
The Critical Role of Regulatory Affairs Specialists in CROs in India
With the rise in the Indian pharmaceutical and biotechnology industries, the role of Regulatory Affairs Specialists (RAS) has become crucial for navigating the current maze-like regulatory environment. Those persons working in Contract Research Organizations (CROs) ensure that regulations are met both at home and abroad to allow drugs to go from the developmental process, clinical trials, and marketing approval phases. The article observes the roles, significance, and problems of Regulatory Affairs Specialists in Indian CROs.
-
 Feb 03
Feb 03
4 Medical Devices Company: Pioneering Healthcare Technology for the Future
With the rapidly evolving discipline of medicine, healthcare equipment is essential to take healthcare forward for patients, treatment, and available treatments. A leading medical device firm uses creativity, technology, and medical knowledge to develop products that help improve healthcare, save lives, and give hope to patients worldwide. This article explores the history, product line, worldwide power, and prospects for a top medical device firm as it dives into its operations, creativity, and vision.
-
 Feb 03
Feb 03
Innovations, Challenges, and the Future of Life Science Companies
Life science enterprises are among the most innovative technologies in the fields of ecology, health, and farming. The companies produce everything ranging from pharmaceutical development and biotechnology research to the manufacture of medical devices and diagnostic equipment. Apart from being crucial to the health industry, the life science business is important for the development of the world economy. Here, the structure, challenges, and potential futures of life science companies are discussed, taking into consideration both their social contributions and the challenges they experience in a rapidly changing environment.
-
 Feb 03
Feb 03
Types of Specimen Collection: Methods and Procedures
Another critical component of medical diagnosis is specimen collection. These are samples obtained from a patient to trace, detect, and diagnose diseases. Reliability in the test depends mainly on how specimens are collected, handled, and preserved. The various specimens may depend on how the specimen needed should be. Some of the specimens include swabs, blood, urine, stool, and tissue. In this regard, every method has certain prerequisites and procedures to be adopted to minimize the chances of contamination by the sample, and this is highly important so that the correct and reliable diagnosis is made through the diagnostic test. Specimen collection plays a greater role than simply being convenient because it directly affects treatment results and even based on the success of treatments.
-
 Feb 03
Feb 03
Sample Collection Procedure: Ensuring Quality and Accuracy
In most of the disciplines, the collection of samples is considered an important aspect in research investigations, medical diagnostics, and environmental testing. The process used in gathering, handling, and processing samples significantly impacts the results obtained. An orderly process of sample collection improves dependable findings, sample integrity, and reduced levels of contamination. This report details the main procedures of sampling concerning the different types of samples, instruments, best practices, and things to consider. We elaborate further on the subtleties of each sample type in the following chapters, discuss the protocols one must follow when collecting them, and further emphasize the importance of sample integrity throughout the entire process.
-
 Feb 03
Feb 03
Clinical Trials in India: An Overview
It is very important for the progress of medical science and the safety of patients. Today, it stands tall as one of the most important sites gaining much importance among clinical trials across the world. It offers a different kind of landscape for research and development for the pharmaceutical world in general and researchers. This blog explores the issues of clinical trials conducted in India, their problems, and prospects for the future in the country. India has attracted a vast pool of participants for clinical trials that could increase the chances for more generalized and applicable results in different populations because it is a country of immense cultural and genetic riches.
-
 Feb 03
Feb 03
The Role of Biobanks in Modern Medicine
Biobanks are critical resources in the collection, storage, and management of biological samples and their related data for purposes of scientific research. They have great value to present-day medicine, which contributes vastly toward better and deeper comprehension of diseases, generation of new treatments, and improvement of public health efforts. This paper covers every aspect of biobanks-from their use in research activities, the moral issues related to applying the concept, to their potential in the future. As reliance on personalized medicine continues to grow, biobanks themselves serve as a source of infrastructure capable of meeting diverse needs for different research, integrating genetic, environmental, and lifestyle data into an all-encompassing view of health and disease.
-
 Feb 03
Feb 03
The Growing Role of Clinical Research Organizations (CROs) in India
India has emerged as one of the biggest players in the global clinical research sphere. The country, with its enormous diversified population and low-cost infrastructure, has seen a boom in Clinical Research Organizations (CROs) here. It assists in every spectrum of drug development, from clinical trials to data management. This article will discuss various types of CROs; how they impact the process of drug development; and their prospects, especially for India. India has emerged as a hub for CROs following the spurt in clinical research activities that led to an increased demand for new treatments and therapies. The attraction of its regulatory setup, which had transformed into making approvals for clinical trials relatively smoother, further increases its appeal as a research hub. As the landscape of clinical research continues to change, CROs are to be expected to change and improve accordingly, embracing new technologies and methodologies while keeping up with what global markets require.
-
 Feb 03
Feb 03
Medical Thesis Writing Services: Supporting Academic and Clinical Research
Medical thesis writing services are very crucial tools that are needed for students, academics, and clinicians inquiring into advanced research. Medical thesis writing services can provide experience with structuring complex research studies, proper formatting, and peer-reviewed literature support. The importance of medical thesis writing services, the scope, and the contribution toward high-quality academic output are some of the topics discussed in this article. In the contemporary competitive landscape of academics, there has never been pressure to come up with original and impactful research. As medical fields are revolutionizing at an incredibly fast rate, rigorous academic standards make it a mandate for researchers and academicians to engage with writing services which shall not only provide them with basic support but give them an all-round understanding of the research process, from conception to publication. The complexity of medical research oftentimes calls for a multidisciplinary approach, which often puts professional writing services in as good a position as anyone in the field.
-
 Feb 03
Feb 03
Urine Sample Collection: Importance in Clinical Diagnostics
The process of urine sample collection becomes routine but essential in medical diagnostics. The samples discovered urine infections, various types of kidney disorders, and so much more. This paper highlights proper urine sample collection and its role in accurate clinical diagnosis, the techniques that accompany it. The knowledge of all these subtleties in the handling of urine is important for health workers and the patients alike, as improper methods may lead to misdiagnosis and ineffective treatment plans.
-
 Feb 03
Feb 03
Blood Sample Collection Procedure: Guidelines and Best Practices
Blood sample collection has been one of the common routine medical services that are applied for diagnostic and follow-up work. Proper techniques in collecting the sample should guarantee integrity both for the sample and the patient. The purpose of the present paper is to define methods for blood sample collection in a clinical setting, instrumentation, and guidelines. Incorrect or contaminated samples may lead to misdiagnosis and inappropriate therapy, therefore healthcare professionals should be trained on proper collection techniques and procedures. Finally, the comfort of a patient's psyche will be during the blood collection procedure, thus a need to have empathy and communication in accomplishing the process.
-
 Feb 03
Feb 03
The Importance of Pre-Clinical Research in Drug Development
The preclinical stage is the most essential research stage because it bridges the laboratory studies with clinical trials. It is a stage of fundamental examination and validation of new therapies prior to their being allowed to encounter the human subjects. This is quite interesting because the blog posting reads quite a lot of information regarding the preclinical research in which it reveals data in the context of its methodology, importance of the field, and also challenges in such studies. Clearly would be understood that the subtlety in preclinical studies has given insights into some level of drug development. To add importance to it, it would relate to the protection of human life and promotion in medical science.
-
 Feb 03
Feb 03
A Comprehensive Guide to Lab Blood Collection
Lab blood collection is one of the most critical healthcare practices. The information availed in diagnosing and follow-up in the management of health disorders can be very vital. In this blog post, we discussed the topic of blood collection: its importance, the process, and best practices for accurate outcomes. So, whether you're a practitioner or a patient, let us de-mystify this all-important process of blood collection.
-
 Feb 03
Feb 03
The Value of Biomedical Companies in Advancing Healthcare
Biomedical companies act as spearheads of innovations in medical fields since they develop new technologies, therapies, and diagnostic tools, and advance many methods for developing healthcare. This article puts out important contributions from biomedical companies, economic impact, challenges they face, and prospects in the health provision changing landscape.
-
 Feb 03
Feb 03
Data Management in Clinical Research: Ensuring Accuracy and Efficiency
In today's world of clinical research, one place where efficiency is imperative is through data management. The increasing complexity of clinical trials and more extensive data production coupled with tougher regulatory requirements necessitate a robust system of managing clinical trial data. Data management involves collecting, storing, and analyzing clinical trial data to ensure that the information is free of errors, valid, and compliant with regulatory standards. This paper discusses the different aspects of data management in clinical research: its significance, challenges, methodologies, and trends in the future.
-
 Feb 03
Feb 03
The Role and Impact of Clinical Trial Organizations in Medical Research
With clinical trials playing such a very key role in providing the country with information on how safe and effective new treatments and drugs are, clinical trial organizations, or CTOs, truly emerge as critical interlinks among the researchers, the pharmaceutical companies, and the regulatory authorities. Hence, the paper discusses different aspects of clinical trial organizations about their composition, roles, and difficulties within the dynamic field of medical research.
-
 Feb 03
Feb 03
A Comprehensive Overview of CDISC
The Clinical Data Interchange Standards Consortium (CDISC) plays a large role in the clinical research arena as it develops standards for collecting, collating, and disseminating data associated with clinical trials. Clinical research, since its increasing complexity, has become an area of high need, with sponsors, researchers, and regulatory bodies all depending on CDISC standards for compliance with regulations. This paper provides an in-depth view of the CDISC guidelines, its merits, problems, and directions for the future.
-
 Feb 03
Feb 03
The Rise of CRO Companies in Hyderabad
Hyderabad has emerged as the nerve center of the pharmaceutical and biotechnology industries, with Contract Research Organizations (CROs) making drug discovery, clinical trials, and regulation a wonder. Here, in this blog, we discuss how CRO companies have thrived in Hyderabad, their services, the advantages offered to sponsors, and some of the challenges in this ever-changing landscape.
-
 Feb 03
Feb 03
The Rise of Clinical Research Companies in India
Skilled manpower, cost-effectiveness, and a facilitative government policy have enabled India to emerge as a thriving hub of clinical research companies. Such companies, which include Contract Research Organizations (CROs) globally, play a critical role in the development process of drugs and pharmaceuticals, as well as in clinical trials for the pharmaceutical, biotechnology, and medical device industries around the world. Here, this blog will discuss the development, significance, services, and challenges that have been faced by clinical research companies in India.
-
 Feb 03
Feb 03
Principles of Specimen Collection
Specimen collection forms the core procedure in both clinical diagnostics and research activities. High-quality specimens that result from an accurate, reliable specimen collection process are necessary for accurate laboratory results. The blog discusses guiding principles in collecting specimens; the implications of these practices and the action steps in the proper handling of specimens to minimize diagnostic errors.
-
 Feb 03
Feb 03
Exploring the Rise and Transformation of the Contract Research Organization (CRO) Industry
The Contract Research Organization or CRO has emerged as a fast-growing and ever-increasingly relevant sector for the pharmaceutical, biotechnology, and medical device industries worldwide for the past few decades. By offering outsourced research services, CROs have helped reduce costs while increasing innovation and speed of drug development. This article discusses historical and current trends, challenges, and opportunities of the CRO industry regarding their importance in the healthcare community today.
-
 Feb 03
Feb 03
Understanding Blood Collection: Techniques, Importance, and Challenges
Blood collection is one of the most vital dimensions of health care since it contributes to diagnosis, research, and even therapeutic interventions. This is one of the important steps toward the basis of correct laboratory results to aid in guiding patient care and treatment. Besides helping out as a diagnostic tool, blood collection helps many people donate and accept transfusions, saving many lives worldwide. The techniques may differ depending on the case, such as the patient's condition and the tests to be done on the patient, like venipuncture, fingerstick, or arterial sampling. Blood collection, in general, is viewed as a routine yet challenging task: there's always a risk of inflicting harm on the patient, the danger of contamination, and complicated difficult venous access complications, to name a few. Proper training, standardized procedures, and advancements in new instruments all contribute to improved acceptance of the said procedures.
-
 Feb 03
Feb 03
The Evolution and Importance of CRO Medical Services in Clinical Research
In recent years, medical and medical industries depend more and more on CROs, which take care of the complexities required in clinical trials and research projects. CRO medical services are now an essential part of helping businesses develop new drugs, devices, and therapies much faster and efficiently. CROs hold a vital function in accelerating medical innovation because of their specialized knowledge, worldwide reach, and comprehension of regulations. With this article, you will have a chance to understand the various aspects of CROs' participation in medical research, issues they solve, and key benefits they offer.
-
 Feb 03
Feb 03
Biorepositories: A Cornerstone of Scientific Advancement
Biobanks, or more directly, biorepositories are institutions that gather, store, and maintain biological specimens for research. Some biological specimens may include tissue, blood, or other body fluids which will help enlarge our horizon of medicine and allow new treatment methods. Researchers, medical science practitioners, and policymakers must be very familiar with biorepositories as the demand for quality biological resources is ever-growing. It considers all aspects of biorepositories: definition, importance, varieties, difficulties, and prospects in the biomedical research field.
-
 Feb 03
Feb 03
Biological Research: Unraveling the Mysteries of Life
Biological research refers to the study of anything that lives and that which surrounds it. It is pretty much a broad category, covering quite a lot about a variety of subjects. This area of study has facilitated many situations in solving issues such as environmental ones, healthcare, and most of all, in understanding basic biological processes. The work in biology research steps forward: advances knowledge of life itself as well as the development of useful applications that could improve both human health and our planet's sustainability. Based on this idea, the article generally addresses different aspects of biological research, especially the importance, method, and new horizons.
-
 Feb 03
Feb 03
The Rise of Contract Research Organizations in India
It has reached a boom position in the pharmaceutical and biotechnology industries in India because of the increasing demands for innovative research and development. Contract Research Organizations have emerged to be essential players in such an arena, providing specialized services in aiding the drug development process. This blog will describe the importance, growth, and service delivery of CROs in India, specifically concerning clinical data management, an integral function that spells all the difference between successful clinical studies and regulatory approval.
-
 Feb 03
Feb 03
Phases of Drug Development: From Discovery to Approval
Drug development is a multi-year process that involves a long chain of processes to ensure that the newly developed drugs are safe and effective for use in the market. This research article goes into the crucial stages involved in drug development, outlines their importance in new drug launches, challenges encountered, and the regulatory aspects involved.
-
 Feb 03
Feb 03
Clinical Research Institutes in Hyderabad: Pioneering Innovation in Drug Development
Known as 'Genome Valley,' Hyderabad is fast gaining importance as a center for clinical research in India, mainly because it houses a very strong pharmaceutical and biotech industry. Ensuring maximization of research and development in the city, numerous clinical research institutes have indeed been set up here, contributing much more to the global healthcare scenario. This blog goes through the importance, growth, services, and challenges faced by the institutes in Hyderabad, highlighting the vital role they play in advancing medical science.
-
 Feb 03
Feb 03
Exploring the Medical Writing Services
Medical writing services have been an integral part of the healthcare and pharmaceutical industries for many years. Such services include preparing scientifically accurate, clear, and compliant documents to support the communication of relevant research findings, clinical trial data, regulatory requirements, and medical education materials to be submitted by regulatory authorities, marketed, or used as education for the patient. This paper explores the features of medical writing services, including the process and the significance of the services to the medical field.
-
 Feb 03
Feb 03
The Role and Importance of Preclinical Contract Research Organizations (CROs) in Drug Development
Preclinical contract research organizations or CROs are significant contributors to the biotech and pharmaceutical industries. It is at this stage, the preclinical stage of drug development when therapeutic candidate compounds are researched for safety, efficacy, and toxicology before moving on to the clinical stages. They offer a wide scope of services in support of this process. Since pharmaceutical firms have unique infrastructures and expertise, they will reduce their risks, maximize their resources, and expedite the development of new drugs. The essay summarizes the role, offerings, and impact of preclinical CROs the greatest challenges they face, and new market innovations
-
 Feb 03
Feb 03
Biometrics: Revolutionizing Security and Identity Managementy
Biometrics is the identification and authentication of people through their biological features including voice, iris patterns, fingerprints, facial recognition, and DNA. Biometrics is fast becoming an indispensable tool in a world where verification of identity and digital security has become existential; it offers better security, efficiency, and accessibility. The article addresses the types of biometric systems, their applications in various industries, the problems and ethical concerns they pose, and the future of biometric technology.
-
 Feb 03
Feb 03
Specimen: A Vital Component in Scientific Discovery and Understanding
A specimen is any sample or living organism collected for scientific study and research. Therefore, Scientists are working around specimens in all research related to biological sciences, medical sciences, archeology, and geology. A specimen provides scientists with the information they need to know, classify, or learn new aspects of the natural world. Examples include tissue samples used in clinical research as well as fossils used in paleontology. Reviewing the importance of specimens in various scientific fields, data collection, and preservation methods, problems associated with the process of specimen handling, and the scope for specimen-oriented research have been discussed in this paper.
-
 Feb 03
Feb 03
Blood Sample Collection: A Comprehensive Guide to Techniques, Importance, and Challenges
Blood sampling is an essential part of medical research and diagnosis. It provides an absolute range of information that is required for laboratory research, diagnosis of disease, and monitoring the health conditions. Blood samples are a vital component of modern health care-from routine checkups to complex genetic tests. This paper discusses the techniques used for the withdrawal of blood samples, the use of blood samples in diagnosis, the troubles faced by the process, and new trends in critical practice.
-
 Feb 03
Feb 03
Sample Collection: Techniques, Importance, and Challenges in Medical Diagnostics
Laboratory access is restricted. A crucial stage in the determination process is sampling, where medical practitioners are in a position to collect biological samples that will be taken for assessment in the laboratory. The samples collected and urinary to biopsy tissue and swabs all provide valuable information about the condition of the patient and contribute towards the diagnosis of several medical conditions. It examines in much detail sample collection methods, their role in diagnosis, the challenges encountered along the way, and the developments that will shape this critical sphere of medicine.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Blood Collection Procedure: Techniques, Guidelines, and Best Practices
Blood collection is part of the health care process in the diagnosis and monitoring of a patient's disease. It also aids in research. A proper way of taking blood ensures that the samples are intact and will help reduce the patient's discomfort. The paper discusses the several approaches toward collecting blood, the steps involved, the importance of protocols followed, and the progress made in this vital practice.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Clinical Data Interchange Standards Consortium (CDISC): Transforming Clinical Research and Data Management
CDISC is the improvement of clinical studies and an increased management of healthcare information. It was formed to develop a global standard for data that is being used in clinical studies to make it easier to interchange and manage the data between researchers, regulatory bodies, and pharmaceutical companies for easier analysis. The article speaks about how important CDISC is, the different standards, how it is affecting clinical trials, and how the route for data interchange will be in the future.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Understanding Blood Collection Methods: Techniques, Procedures, and Best Practices
Blood collection is one of the procedures one does in diagnosing, as well as treatment and follow-up care of many medical conditions. This report discusses different methods applied for blood collection techniques, from the applications, procedures, and advantages, to potential disadvantages. Healthcare providers ought to know the methods so blood can be collected correctly and safely, thus improving the outcome for patients eventually.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Clinical Research Near Me
Clinical research is one of the most important mechanisms by which medicine evolves and new treatments are discovered. Clinical research represents one of the choices through which individuals can receive access to new treatment while contributing to scientific advancement. The article explores the concept of clinical research, the benefits, finding a clinical trial near you, and factors to consider during participation.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role and Impact of Healthcare Technology Companies in Modern Medicine
Healthcare technology companies redefine the way we see treatment as far as the services to be offered are concerned. They enhance patient advantages, de-congest their medical workflows, and make health services more accessible to everyone through adopting some of the best digital technologies coupled with innovative solutions in the health care system. From AI diagnosis to telemedicine, these companies are one of those firms that stand at the forefront of changing traditional features in medical practices into more data-driven and streamlined operations. This article will explore various sectors of healthcare technology, some leaders in the field, and their contribution to change.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role and Evolution of Clinical Trial Services in Modern Healthcare
Such clinical trial services are thus also essential in order for new medicines to be developed and for medical science to be given the impetus to progress. The activities basically involve designing the trial, collecting data, regulatory compliance, and patients' recruitment. With rising complexity of illnesses and higher regulatory requirements, these services all the more significantly sustain medicine. In this essay, we will note the different aspects of clinical trial services, their role in drug discovery, and the challenges they face in contemporary healthcare.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Understanding Pharmaceutical Contract Research Organizations (CROs)
Contract research organizations are now becoming integral parts of the ever-changing pharmaceutical business and are enabling an easier streamlining of procedures that produce drugs. Their areas of involvement enable pharmaceutical and biotechnology companies to operate effectively, minimize risks that come with the development of drugs, and also speed up the time to market. This paper aims to discuss pharmaceutical CROs in terms of what they do, their relevance, functions, and future role in the industry.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role of Clinical Trials in Las Vegas
Known well for nightlife and entertainment, Las Vegas is fast becoming a growth hub for medical innovations. This article discusses the role of clinical trials in the development of healthcare, why they're becoming increasingly popular for medical research, the progress made in conducting these trials, and the challenges along the way.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Blood Collection Procedure: Techniques, Guidelines, and Best Practices
The preclinical stage is a key step in drug development and creates a basis for all subsequent clinical studies. This stage mainly seeks to understand the safety, efficacy, and potential risks of a new drug compound before giving it to humans. Any intensive research in the preclinical stage should generate enough information to guide decisions on whether or not a candidate drug is ready for human tests. This article aims to detail the importance, techniques, and legal requirements of preclinical research and the difficulties of researchers in this field.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role and Impact of Biomedical Device Companies in Healthcare
Companies operating in the biomedical device sector represent one of the fronts of innovation in healthcare provision. They have solutions that give highly improved patient outcomes. They specialize in the design and manufacture of medical devices to diagnose, prevent, monitor, and treat various diseases. This paper discourses on the contribution of biomedical device companies to modern-day health care, their growth trajectory, the services offered, as well as the challenges that they come with.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Rise of Regulatory Specialists in India's Contract Research Organizations
As the pharmaceutical and biotechnology industries boom in India, Contract Research Organizations (CROs) are taking on a critical role in hastening the process of drug development. Of the many functions available in such organizations, regulatory specialists have emerged as priceless contributors. The purpose of this paper is to examine the importance of, roles of, and challenges presented by regulatory specialists in CROs in India, their impact on the pharmaceutical landscape, and their contribution to compliance and innovation.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Clinical Research Specialists: Pioneers in Medical Innovation
Clinical research specialists are very integral to the betterment of health care, as they make theoretical medical research more closely linked with practical clinical applications. They work at the top of the medical drug development stage the stage in which they test various medical devices and then develop the application protocols of newly discovered treatments. Thus, this new therapy is not only effective but also very safe for public use. This article looks at the role and responsibility, skills, challenges, and prospects of clinical research specialists.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Importance of Clinical Care Solutions in Modern Healthcare
Clinical care solutions are so adapted that today in the fast-changing healthcare landscape, these are required for enhancing patient outcomes, improving operational efficiency, and delivering high-quality care. The article focuses on the role of clinical care solutions, its benefits to the healthcare provider and patient, the different types of solutions available, and the challenges in implantation in diverse healthcare settings.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role of Biobanking in Modern Healthcare and Research
Biobanking is fast becoming the bedrock of medical science and healthcare development. During a time when demand for precision medicine is growing, biobanks have become valuable assets or tools for scientists and healthcare professionals as research tools in finding diseases, developing new treatments, or tailoring treatments according to specific characteristics. The article focuses on the importance, functioning, challenges, and future potential of biobanks in the rapidly changing arena of health care.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Importance of Urine Samples in Medical Diagnostics
For a long, medical diagnostics has considered the liquid sample of urine as an invaluable tool in understanding the patient's health condition. Since the sample is non-invasive and easily obtained, urine provides information about the metabolic, renal, and urinary systems of the body. This paper presents the importance of urine samples, conditions diagnosed through them, methods of urine collection, methods of analysis, and future directions for urine diagnostics in modern medicine.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Role and Importance of Sample Collection Tubes in Medical Diagnostics
Sample collection tubes are part and parcel of the medical and diagnostics field, forming the crux of the process that takes care of biological specimens' integrity. It is used for collecting, carrying, and storing blood as well as urine, among other body fluids, into the laboratory for tests. Medical diagnostics depend much on the quality of reliability maintained through the process of collecting samples. The paper will attempt to discuss the types, importance, and challenges of sample collection tubes and innovations in such.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Principles of Specimen Collection in Healthcare
Specimen collection is one of the cardinal steps in medical diagnostics, being the bedrock of accurate laboratory tests and clinical decisions. The kind of specimen being taken, urine, tissue, or any other form of biological material should abide by the standard principles such that the results produced shall be sound and valid. This piece reports on the basic principles guiding the collection of specimens, methods applied in practice, the problems associated with the practice, and the role that health practitioners play in incorporating quality standards.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Urine Specimen Collection Methods: Best Practices and Considerations
Proper sample collection is a crucial feature in any medical diagnostics and research work. It is for this reason that all appropriate methods of urine specimen collection should be followed to ensure the success of any test, whether a routine urinalysis, drug test or specific tests for infection and other metabolic diseases. The present paper discusses the various techniques of urine sample collection, their uses, and best practices to ensure an outcome that is accurate and reliable.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Vital Role of Clinical Research Coordinators in Advancing Medical Science
Clinical research coordinators are an important linkage between the researcher, medical practitioner, and study participants. They form the backbone of clinical research as they ensure that clinical studies are conducted efficiently, ethically, and by legal requirements. This article looks into the roles and competencies of CRCs and their importance to the progress of clinical research in terms of advancing medical science.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Evolving Landscape of Medical Device Manufacturers in India
The Indian medical device industry has been registering remarkable growth. This sector gains momentum with technological advancement, growing healthcare needs, and governmental policies to support this growth. Medical device manufacturers play an important part in this industry. This involves innovation which improves not only the level of care for patients but also health care delivery. The blog below looks at the importance, growth, services, and challenges faced by medical device manufacturers in India.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
The Value of Clinical Development
Clinical development is one of the most critical stages in the drug or medical device life cycle. Phase The phase must undergo rigid trials as preliminary testing before the products can reach the market. Clinical Development Value According to the author, clinical development has multifaceted value: the importance of clinical development in the pharmaceutical and biotechnology industries; the responsibility of different stakeholders; and regulatory frameworks.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Clinical Research Jobs in Hyderabad for Freshers
Hyderabad has become a center of clinical research very quickly; several thousands of hospitals, research centers, and educational institutes keep a thriving pharmaceutical industry going on their back. Some of the fresh graduates who just joined the industry here in Hyderabad include joining Contract Research Organizations (CROs) or working with major pharmaceutical corporations. The scope of practice, requirements, and job opportunities for entry-level professionals in clinical research in Hyderabad are discussed in the article.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Top Clinical Research Companies in India
Pharmaceutical and biotechnology companies heavily depend on clinical research organizations to assist them in the process of developing a drug. A large number of CROs exist in India, which offer quality services, cover the entire globe, and hold strong morality in the fast-growing clinical trial market of India. These companies provide services under post-marketing surveillance and early-stage clinical trials. This article discusses the best clinical research firms in India and what contributions they make to the sector.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Understanding Blood Collection and Storage
Current medical procedures involve blood collection and storage. Blood transfusion is used to save lives during operations in critical injuries and even during the treatment of many diseases like anemia and cancer. Proper techniques of blood collection and storage help in the survival and safety of patients. The paper presents the concepts and methodology behind the collection of blood, storage of blood methodologies as well as how these procedures became commonplace around the world in healthcare systems.
-
 Feb 03BloodSampleCollection102
Feb 03BloodSampleCollection102
Clinical Trials Near Me: A Guide to Participation, Benefits, and Challenges
Clinical trials are an important part of medical research. That is because they offer participants the possibility to forward the process of health care while often given advanced therapies. If you are looking for clinical trials in your area, you should know each aspect involved how to find them, and the requirements for eligibility down to possible disadvantages of enrolling. Such an article could provide you with a comprehensive source of guidance in finding and participating in clinical trials in your local region.



